Spectrum Health Structural Heart and Valve Center
The Spectrum Health Structural Heart & Valve Center brings groundbreaking advances to the treatment of structural heart and valve diseases. The center offers new options for valve replacement and repairs as well as treatments for septal defects and stroke prevention for atrial fibrillation. Our specialists have years of experience performing these minimally invasive procedures.
Spectrum benefits from a thorough and collaborative multidisciplinary team of interventional cardiologists, cardiac surgeons, and cardiovascular imaging specialists. We partner to develop individualized care plans while continuously working together to provide patients with innovative and effective cardiovascular care.
Spectrum Health started performing transcatheter aortic valve replacement (TAVR) procedures in 2011. We are a nationally recognized institution due to our advanced technologies and treatment options. We offer several FDA-approved, minimally invasive, catheter-based therapies, as well as clinical trials that provide up and coming treatments. Our state-of-the-art facilities, cutting edge technology and dedicated providers lead to better outcomes for our patients.
Minimally invasive procedures allow for easier and more rapid recovery as well as shorter lengths of stay.
Brain heart
Spectrum Health is one of the only sites nationwide with a well-established Neuro-Cardio board called Brain Heart. Our structural heart and stroke neurology team collaborate closely monthly to identify the appropriate work up for patients with embolic stroke of undetermined source and to identify and describe appropriate treatment options for patients with heart rhythm disorders who are not candidates for anticoagulation.
Hybrid operating rooms
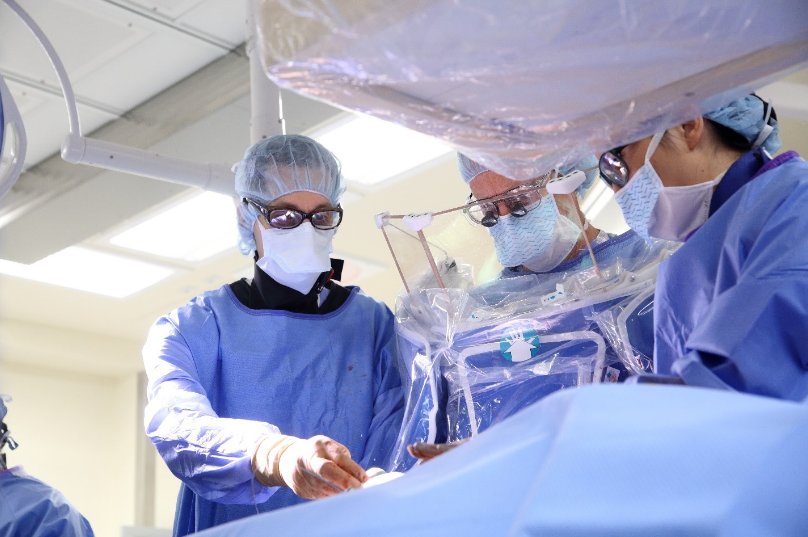
Corewell Health Grand Rapids Hospitals Fred & Lena Meijer Heart Center utilizes hybrid operating rooms for heart valve disease patients. These rooms offer high-end angiographic equipment that is traditionally used in the Cath lab, and they can be used in a surgical environment as well.
Hybrid rooms allow us to use a combination of surgical and percutaneous techniques to provide optimal therapy for the patients and allow for emergent open-heart surgery, if necessary. The Meijer Heart Center cardiovascular operating room hub has been recognized as one of the most efficient operating room hubs in the nation.
Research
Early Feasibility Study (EFS) of the Intrepid ™ TMVR Transseptal System
The prospective, multi-center, non-randomized EFS will evaluate the safety and performance of the Intrepid TMVR System with the transfemoral approach in patients with severe, symptomatic mitral regurgitation (MR) who are ineligible for conventional mitral valve surgery.

The study will enroll and treat up to 15 patients who require mitral valve intervention at up to ten centers. Treated patients will be followed at one, three, six and 12 months, and every six months thereafter through 5 years. The results from this EFS study will inform the development of next generation transfemoral TMVR technologies
Contact Us
For more information or to schedule an appointment
